Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes from combining glycerol and aldehyde, as glyceraldehyde is glycerol with one alcohol group oxidized to an aldehyde.[4]
Structure
Glyceraldehyde has one chiral center and therefore exists as two different enantiomers with opposite optical rotation:
- In the D/L nomenclature, either D from Latin Dexter meaning "right", or L from Latin Laevo meaning "left"
- In the R/S nomenclature, either R from Latin Rectus meaning "right", or S from Latin Sinister meaning "left"
| D-glyceraldehyde (R)-glyceraldehyde (+)-glyceraldehyde |
L-glyceraldehyde (S)-glyceraldehyde (−)-glyceraldehyde | |
| Fischer projection | 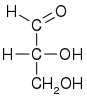
|
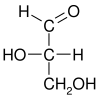
|
| Skeletal formula | 
|

|
While the optical rotation of glyceraldehyde is (+) for R and (−) for S, this is not true for all monosaccharides. The stereochemical configuration can only be determined from the chemical structure, whereas the optical rotation can only be determined empirically (by experiment).
It was by a lucky guess that the molecular D- geometry was assigned to (+)-glyceraldehyde in the late 19th century, as confirmed by X-ray crystallography in 1951.[5]
Aqueous and concentrated solutions of glyceraldehyde
The description above focuses on classification of isomers, but the glyceraldehyde is subject to a further complications: the tendency of hydroxy-aldehydes to exist as hydrates. NMR measurements indicate that in aqueous solution, glyceraldehyde exists in a hydrate owing to this reaction:
- HOCH2CH(OH)CHO + H2O ⇌ HOCH2CH(OH)CH(OH)2
The same study indicates that concentrated ("syrupy") forms of glyceraldehyde exist as dimers, indicating hemiacetal formation.[6]
Nomenclature
In the D/L system, glyceraldehyde is used as the configurational standard for carbohydrates.[7] Monosaccharides with an absolute configuration identical to (R)-glyceraldehyde at the last stereocentre, for example C5 in glucose, are assigned the stereo-descriptor D-. Those similar to (S)-glyceraldehyde are assigned an L-.
Synthesis and reactions
Glyceraldehyde can be prepared from acetals of acrolein (CH2=CHCHO) in two steps, oxidation[8] followed by hydrolysis of the acetal.[9] Its cyclohexylidene acetal can also be produced by oxidative cleavage of the bis(acetal) of mannitol.[10]
Glyceraldehyde is a precursor to four-carbon sugars (tetroses) via cyanation followed by hydrolysis of the cyanohydrin:[4]
- HOCH2CH(OH)CH(OH)CN + 2 H2O → HOCH2CH(OH)CH(OH)CO2H + NH3
- HOCH2CH(OH)CH(OH)CO2H + 2 H2 → HOCH2CH(OH)CH(OH)CH2OH + H2O
Biochemistry
The enzyme glycerol dehydrogenase (NADP+) has two substrates, glycerol and NADP+, and 3 products, D-glyceraldehyde, NADPH and H+.[11]
The interconversion of the phosphates of glyceraldehyde (glyceraldehyde 3-phosphate) and dihydroxyacetone (dihydroxyacetone phosphate), catalyzed by the enzyme triosephosphate isomerase, is an intermediate step in glycolysis.
See also
References
- ^ https://iupac.qmul.ac.uk/BlueBook/P10.html#t1002
- ^ https://iupac.qmul.ac.uk/BlueBook/P10.html#t1002
- ^ Merck Index, 11th Edition, 4376
- ^ a b "Molecule of the Week: D-Glyceraldehyde". C&E News. 2023.
- ^ Bijvoet, J. M.; Peerdeman, A. F.; Van Bommel, A. J. (1951). "Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays". Nature. 168 (4268): 271–272. Bibcode:1951Natur.168..271B. doi:10.1038/168271a0.
- ^ Angyal, SJ; Wheen, RG (1980). "The Composition of Reducing Sugars in Aqueous Solution : Glyceraldehyde, Erythrose, Threose". Australian Journal of Chemistry. 33 (5): 1001. doi:10.1071/CH9801001.
- ^ "22.03: The D and L Notation". Chemistry LibreTexts. 2015-03-19. Retrieved 2022-01-09.
- ^ Witzemann, E. J.; Llloyd Evans, Wm.; Hass, Henry; Schroeder, E. F. (1931). "dl-Glyceraldehyde Ethyl Acetal". Organic Syntheses. 11: 52. doi:10.15227/orgsyn.011.0052.
- ^ Witzemann, E. J.; Llloyd Evans, Wm.; Hass, Henry; Schroeder, E. F. (1931). "dl-Glyceraldehyde". Organic Syntheses. 11: 50. doi:10.15227/orgsyn.011.0050.
- ^ Dhatrak, N. R.; Jagtap, T. N.; Shinde, A. B. (2022). "Preparation of 1,2:5,6-Di-O-cyclohexylidene-D-mannitol and 2,3-Cyclohexylidene-D-glyceraldehyde". Organic Syntheses. 99: 363–380. doi:10.15227/orgsyn.099.0363. S2CID 254320929.
- ^ Kormann, Alfred W.; Hurst, Robert O.; Flynn, T.G. (1972). "Purification and properties of an NADP+-dependent glycerol dehydrogenase from rabbit skeletal muscle". Biochimica et Biophysica Acta (BBA) - Enzymology. 258 (1): 40–55. doi:10.1016/0005-2744(72)90965-5. PMID 4400494.













You must be logged in to post a comment.