Chemical compound
Pharmaceutical compound
Prorenone (developmental code name SC-23133 ) is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed.[ 1] lactonic form of prorenoic acid (prorenoate), and prorenoate potassium (SC-23992), the potassium salt of prorenoic acid, also exists.[ 1] potent than spironolactone as an antimineralocorticoid in animals, and it may act as a prodrug to prorenone.[ 1] mineralocorticoid receptor , prorenone also binds to the glucocorticoid , androgen , and progesterone receptors .[ 2] [ 3] in vivo [ 3] inhibitor of aldosterone biosynthesis .[ 4]
Chemistry
Synthesis
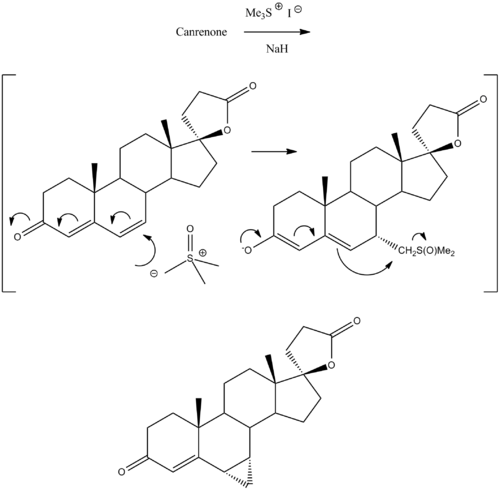
Prorenone can be synthesized via a Johnson–Corey–Chaykovsky reaction by reaction of canrenone with trimethylsulfoxonium iodide and sodium hydride .[ 5]
See also
References
^ a b c Claire M, Rafestin-Oblin ME, Michaud A, Roth-Meyer C, Corvol P (April 1979). "Mechanism of action of a new antialdosterone compound, prorenone". Endocrinology . 104 (4): 1194– 1200. doi :10.1210/endo-104-4-1194 . PMID 436757 . ^ Szasz G, Budvari-Barany Z (19 December 1990). Pharmaceutical Chemistry of Antihypertensive Agents ISBN 978-0-8493-4724-5 . ^ a b Kamata S, Matsui T, Haga N, Nakamura M, Odaguchi K, Itoh T, et al. (September 1987). "Aldosterone antagonists. 2. Synthesis and biological activities of 11,12-dehydropregnane derivatives". Journal of Medicinal Chemistry . 30 (9): 1647– 1658. doi :10.1021/jm00392a022 . PMID 3040999 . ^ Netchitailo P, Delarue C, Perroteau I, Leboulenger F, Capron MH, Vaudry H (January 1985). "Relative inhibitory potency of five mineralocorticoid antagonists on aldosterone biosynthesis in vitro". Biochemical Pharmacology . 34 (2): 189– 194. doi :10.1016/0006-2952(85)90123-6 . PMID 2981534 . ^ US 3845041 , Chinn L, "7-Halomethyl-17-hydroxy-3-oxo-17alpha-pregn-4-ene-21-carboxylic acid gamma-lactones", issued 19 October 1974, assigned to GD Searle LLC.
AR Tooltip Androgen receptor
Agonists SARMs Tooltip Selective androgen receptor modulator Antagonists
GPRC6A
MR Tooltip Mineralocorticoid receptor
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )









You must be logged in to post a comment.