4-Nitrobenzoic acid is an organic compound with the formula C6H4(NO2)CO2H. It is a pale yellow solid. It is a precursor to 4-nitrobenzoyl chloride, the precursor to the anesthetic procaine and folic acid. It is also a precursor to 4-aminobenzoic acid.[6]
Production
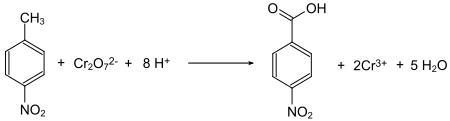
4-Nitrobenzoic acid is prepared by oxidation of 4-nitrotoluene using oxygen or dichromate as oxidants.[7]
Alternatively, it has been prepared by nitration of polystyrene followed by oxidation of the alkyl substituent. This method proceeds with improved para/ortho selectivity owing to the steric protection of the ortho positions by the polymer backbone.
Safety
This compound has a rat LD50 of 1960 mg/kg.[8]
References
- ^ "4-nitrobenzoic acid - PubChem Public Chemical Database". Retrieved 11 April 2010.
- ^ a b c d "Safety data for p-nitrobenzoic acid". Archived from the original on 27 May 2008. Retrieved 11 April 2010.
- ^ "p-Nitrobenzoic acid". Archived from the original on 7 May 2010. Retrieved 11 April 2010.
- ^ "Dissociation Constants Of Organic Acids And Bases". Retrieved 11 April 2010.
- ^ "Bordwell pKa Table (Acidity in DMSO)". Retrieved 11 April 2010.
- ^ Takao Maki; Kazuo Takeda (2002). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 3527306730..
- ^ O. Kamm; A. O. Matthews (1922). "p-Nitrobenzoic Acid". Org. Synth. 2: 53. doi:10.15227/orgsyn.002.0053.
- ^ "Material Safety Data Sheet - P-nitrobenzoic acid MSDS". Archived from the original on 7 August 2011. Retrieved 11 April 2010.









You must be logged in to post a comment.