Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
Synthesis and occurrence
Azetidines can be prepared by reduction of azetidinones (β-lactams) with lithium aluminium hydride. Even more effective is a mixture of lithium aluminium hydride and aluminium trichloride, a source of "AlClH2" and "AlCl2H".[3] Azetidine can also be produced by a multistep route from 3-amino-1-propanol.[4]
Regio- and diastereoselective synthesis of 2-arylazetidines could be performed from appropriately substituted oxiranes via ring transformation. It is controlled by Baldwin's Rules with remarkable functional group tolerance.[citation needed]
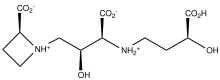
Azetidine and its derivatives are relatively rare structural motifs in natural products. They are a component of mugineic acids and penaresidins. Perhaps the most abundant azetidine containing natural product is azetidine-2-carboxylic acid - a toxic mimic of proline.[5]
See also
- Azete, the unsaturated analog
References
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- ^ Alcaide, Benito; Almendros, Pedro; Aragoncillo, Cristina (2007). "Β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products". Chemical Reviews. 107 (11): 4437–4492. doi:10.1021/cr0307300. PMID 17649981.
- ^ Donald H. Wadsworth (1973). "Azetidine". Organic Syntheses. 53: 13. doi:10.15227/orgsyn.053.0013.
- ^ Kovács, Ervin; Ferenc, Faigl; Zoltan, Mucsi (Aug 10, 2020). "Regio- and Diastereoselective Synthesis of 2-Arylazetidines. Quantum Chemical Explanation of Baldwin's Rules for the Ring-formation Reactions of Oxiranes". Journal of Organic Chemistry. 85 (17): 11226–11239. doi:10.1021/acs.joc.0c01310. PMC 7498157. PMID 32786621.








