
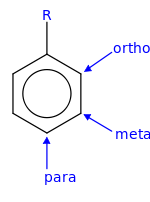
In organic chemistry, the phenylene group (−C6H4−) is based on a di-substituted benzene ring (arylene). For example, poly(p-phenylene) is a polymer built up from para-phenylene repeating units.[1] The phenylene group has three structural isomers, based on which hydrogens are substituted: para-phenylene, meta-phenylene, and ortho-phenylene.
References
- ^ p. C-9, Section 11.6, Handbook of Chemistry and Physics, 62nd Edition, 1981-1982, CRC Press








