Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula C
3H
6O, having a four-membered ring with three carbon atoms and one oxygen atom.
The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring.
Production
A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C:[2]

Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products including water, potassium chloride, and potassium acetate.
Another possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization[3] as well as through decarboxylation of a six-membered cyclic carbonate.[citation needed]
Derivatives
More than a hundred different oxetanes have been synthesized.[citation needed] Functional groups can be added into any desired position in the oxetane ring, including fully fluorinated (perfluorinated) and fully deuterated analogues. Major examples are:
| Name | Structure | Boiling point, Bp [°C] |
|---|---|---|
| 3,3-Bis(chloromethyl)oxetane | 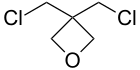
|
198[4] |
| 3,3-Bis(azidomethyl)oxetane | 
|
165[5] |
| 2-Methyloxetane | 
|
60[citation needed] |
| 3-Methyloxetane | 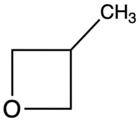
|
67[citation needed] |
| 3-Azidooxetane | 
|
122[6] |
| 3-Nitrooxetane | 
|
195[7] |
| 3,3-Dimethyloxetane | 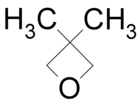
|
80[citation needed] |
| 3,3-Dinitrooxetane | 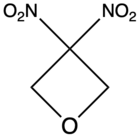
|
– |
Taxol

Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment.[8] The attached oxetane ring is an important feature that is used for the binding of microtubules in structure activity; however little is known about how the reaction is catalyzed in nature, which creates a challenge for scientists trying to synthesize the product.[8]
Reactions
Oxetanes are less reactive than epoxides, and generally unreactive in basic conditions,[9] although Grignard reagents at elevated temperatures[10] and complex hydrides will cleave them.[11] However, the ring strain does make them much more reactive than larger rings,[12] and oxetanes decompose in the presence of even mildly acidic nucleophiles.[13] In non-nucleophilic acids, they mainly isomerize to allyl alcohols.[14]
Noble metals tend to catalyze isomerization to a carbonyl.[15]
In industry, the parent compound, oxetane polymerizes to polyoxetane in the presence of a dry acid catalyst,[16] although the compound was described in 1967 as "rarely polymerized commercially".[17]
See also
- β-Propiolactone or 2-oxetanone.
- 3-Oxetanone
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ C. R. Noller (1955). "Trimethylene Oxide". Organic Syntheses. 29: 92; Collected Volumes, vol. 3, p. 835.
- ^ Patai 1967, pp. 411–413.
- ^ "78-71-7 CAS MSDS (3,3-BIS(CHLOROMETHYL)OXETANE) Melting Point Boiling Point Density CAS Chemical Properties". www.chemicalbook.com. Retrieved 2022-12-28.
- ^ Akhtar, Tauseef; Berger, Ronald; Marine, Joseph E; Daimee, Usama A; Calkins, Hugh; Spragg, David (2020-08-13). "Cryoballoon Ablation of Atrial Fibrillation in Octogenarians". Arrhythmia & Electrophysiology Review. 9 (2): 104–107. doi:10.15420/aer.2020.18. ISSN 2050-3377. PMC 7491081. PMID 32983532.
- ^ Baum, Kurt; Berkowitz, Phillip T.; Grakauskas, Vytautas; Archibald, Thomas G. (September 1983). "Synthesis of electron-deficient oxetanes. 3-Azidooxetane, 3-nitrooxetane, and 3,3-dinitrooxetane". The Journal of Organic Chemistry. 48 (18): 2953–2956. doi:10.1021/jo00166a003. ISSN 0022-3263.
- ^ "3-Nitrooxetane | C3H5NO3 | ChemSpider". www.chemspider.com. Retrieved 2022-12-28.
- ^ a b Willenbring, Dan; Tantillo, Dean J. (April 2008). "Mechanistic possibilities for oxetane formation in the biosynthesis of Taxol's D ring". Russian Journal of General Chemistry. 78 (4): 723–731. doi:10.1134/S1070363208040336. S2CID 98056619.
- ^ Patai 1967, p. 425.
- ^ Patai 1967, pp. 63, 425.
- ^ Patai 1967, pp. 67–68.
- ^ Patai 1967, pp. 376–377.
- ^ Patai, Saul, ed. (1967). The Chemistry of the Ether Linkage. The Chemistry of Functional Groups. London: Interscience / William Clowes and Sons. pp. 28–30. LCCN 66-30401.
- ^ Patai 1967, p. 696.
- ^ Patai 1967, pp. 697, 700.
- ^ Penczek & Penczek (1963), "Kinetics and mechanism of heterogeneous polymerization of 3,3-bis(chloromethyl)oxetane catalyzed by gaseous BF3" in Die Makromolekuläre Chemie. Wiley.
- ^ Patai 1967, p. 380.









You must be logged in to post a comment.