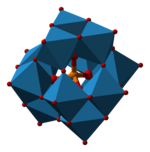
Ammonium phosphomolybdate is the inorganic salt of phosphomolybdic acid with the chemical formula (NH4)3PMo12O40. The salt contains the phosphomolybdate anion, a well known heteropolymetalate of the Keggin structural class.
Synthesis
Ammonium phosphomolybdate can be made by heating ammonium orthomolybdate combined with phosphoric acid and nitric acid, yielding ammonium nitrate, water, and a yellow precipitate of ammonium phosphomolybdate is obtained.[3]
- 12 (NH4)6Mo7O24(H2O)4 + 7 Na2HPO4(H2O) + 65 HNO3 → 7 (NH4)3Mo12PO40 + 51 NH4NO3 + 14 NaNO3 + 91 H2O
Normally, it often exists as a hexahydrate, a dark yellow fine crystal which is poorly soluble in water.[3]
It is also obtained as a test result for phosphate ions.
References
- ^ ChemicalBook: http://www.chemicalbook.com/ChemicalProductProperty_EN_CB6162083.htm
- ^ a b "Ammonium phosphomolybdate hydrate".
- ^ a b Dias, José Alves; Dias, Sílvia Cláudia Loureiro; Caliman, Ednéia (2014). "Keggin Structure Polyoxometalates". Inorganic Syntheses: Volume 36. Vol. 36. pp. 210–217. doi:10.1002/9781118744994.ch39. ISBN 9781118744994.









You must be logged in to post a comment.