In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of [C
5H
5]−
and abbreviated as Cp−.[1] It is formed by the deprotonation of cyclopentadiene. The cyclopentadienyl anion is a ligand which binds to a metal in organometallic chemistry.[2]
The first salt with this anion, potassium cyclopentadienide, was prepared by Johannes Thiele in 1901[3] but there wasn't much interest in the topic until the discovery of ferrocene in the 1950s.
Resonance and aromaticity
The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. Each double bond and lone pair provides 2 π-electrons, which are delocalized into the ring.[4]
Cyclopentadiene has a pKa of about 16. It is acidic relative to many carbon acids. The enhanced acidity is attributed to stabilization of the conjugate base, cyclopentadienyl anion.
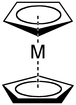
Ligand
Cyclopentadienyl anions form a variety of cyclopentadienyl complexes.[5]
See also
- Cyclopentadienyl radical, [C
5H
5]• - Cyclopentadienyl cation, [C
5H
5]+ - Cyclooctatetraenide anion, [C
8H
8]2−
References
- ^ "Cyclopentadienide". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 14 April 2016.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 66, ISBN 978-0-471-72091-1
- ^ Johannes Thiele (January 1901). "Ueber Abkömmlinge des Cyclopentadiëns". Berichte der Deutschen Chemischen Gesellschaft (in German). 34 (1): 68–71. doi:10.1002/CBER.19010340114. ISSN 0365-9496. Wikidata Q126217369.
- ^ Paul, Satadal; Goswami, Tamal; Misra, Anirban (2015-10-01). "Noncomparative scaling of aromaticity through electron itinerancy". AIP Advances. 5 (10): 107211. Bibcode:2015AIPA....5j7211P. doi:10.1063/1.4933191.
- ^ C. Elschenbroich (2006). Organometallics. VCH. ISBN 978-3-527-29390-2.










You must be logged in to post a comment.